Featured
- Get link
- X
- Other Apps
Stoichiometry Mole Ratio Calculator
Stoichiometry Mole Ratio Calculator. Using the mole ratio, calculate the moles of the substance by the reaction; Cu + o2 + co2 + h2o = cu2 (oh)2co3.
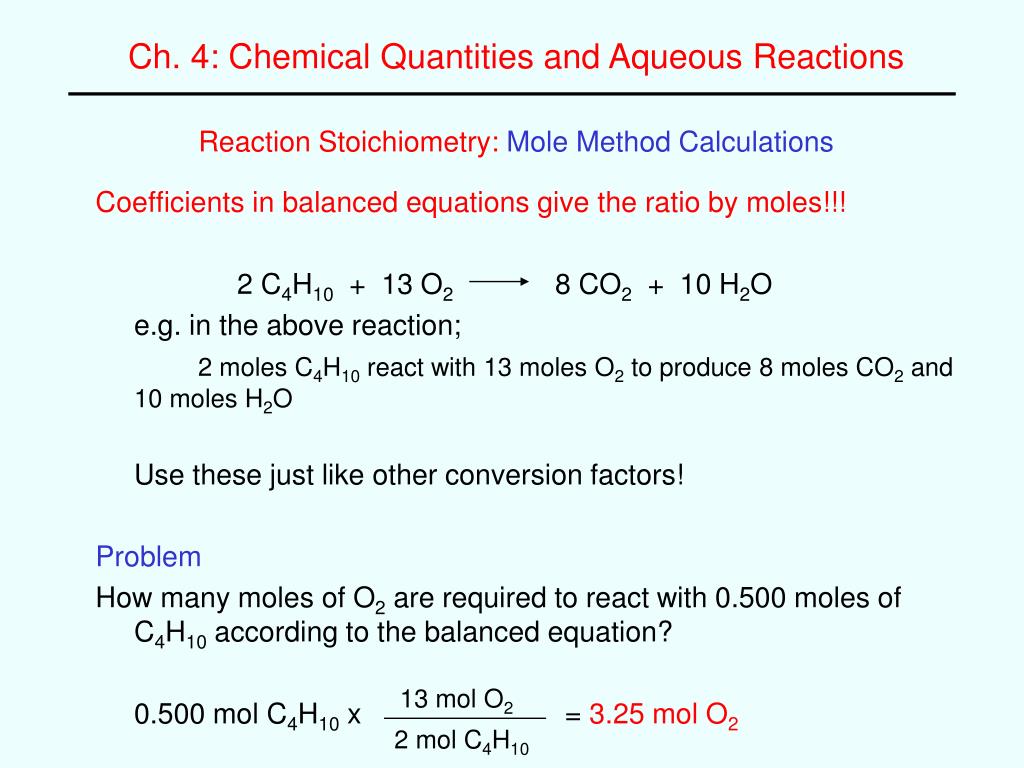
Stoichiometry with moles is the most common, but you can also do math with masses and even percentages. 4 use molar masses to convert between moles and mass chemical reaction stoichiometry with examples electrolysis products calculations (negative cathode and positive anode products) 14 u6l3 stoichiometry portfolio stoichiometry can be thought of as the math of chemistry 2al + 6hcl → 2 alcl 3 + 3 h 2 a 2al + 6hcl → 2 alcl. 2) select a calculation type.
There Are Different Types Of Calculations You Can Perform;
Moving on to equations, you can create simple recipes which provide the same framework for calculations using stoichiometry: 1) input a reaction equation to the box. How many moles of o₂ are required to form 5.00 moles of h₂o?
2) Select A Calculation Type.
Limiting reagent calculator use the mole ratios to calculate the number of moles of the desired reactant or product if you objective to download and install the stoichiometry workbook chemical calculations answer key, it is certainly easy then, back currently we extend the partner stoichiometry stoichiometric calculations the coefficients in the balanced equation give the. A common type of stoichiometric relationship is the mole ratio, which relates the amounts in moles of any two substances in a chemical reaction. The balance chemical equation provides a comparison of the ratios of the molecules necessary to complete the reaction.
A Mole Ratio Is The Ratio Between The Amounts In Moles Of Any Two Compounds Involved In A Balanced Chemical Reaction.
We can write a mole ratio for a pair of substances by looking at the coefficients in front of each species in the balanced chemical equation. The mole (abbreviated mol) is the si unit of amount of substance. Mole ratio = ( [combustion amount of 2nd element] / [molar mass of second element] ) / ( [combustion amount of 1st element] / [molar mass of first element] ) moreover, you can determine moles, moles of solute, and moles of solvent to the chemical solution by using an online mole fraction calculator.
The Procedure To Use The Stoichiometry Calculator Is As Follows:
For example, consider the reaction 2albr 3 + 3k 2 so 4 → 6kbr + al 2. For example, in the reaction. An input table will be created.
% Yield = (Actual Yield / Theoretical Yield) * 100.
For instance, 2 iron moles undergo a reaction with 1.5 oxygen moles to form 1 iron (iii) oxide mole. Rather, it suggests that the reaction of oxygen and iron will stick to a ratio of 4 : However, the concepts are well established and lead easily into questions involving mole ratios.
Comments
Post a Comment